The microbiome, the collection of bacteria that colonizes your entire digestive tract is incredibly complex. There are so many things to consider when trying to build a healthy, diverse microbiome, but why build a diverse microbiome? A diverse microbiome is clearly associated with health, and a disturbed microbiome is found in many health conditions such as inflammatory bowel diseases, autism, Type 2 diabetes, and depression. At this point it is clear as to how to build a healthy microbiome. In a presentation at the Gut Microbiota for Health World Summit, experts presented what we currently know. A diet high in fruit and vegetables provides ample substrate to build a diverse microbiome while antibiotic use, being delivered by C-sections, frequent use of NSAIDS, and not being breastfed are all factors that can lead to a less diverse microbiome.
The effects of antibiotics on the microbiome were also discussed in relation to weight gain as there is a clear association between antibiotic use in childhood and weight gain later on in life. A study looking at Crohn's disease identified the specific strains of bacteria that are affected in the disease, and as the abundance of specific strains of beneficial bacteria becomes reduced, the abundance of pathogenic strains begins to increase. While antibiotics are frequently used to help combat Crohn's, they may be contraindicated because they are not selective in which bacteria they effect, and reducing the number of beneficial bacteria may have the same effect as increasing pathogenic bacteria by removing competition. Since this study indicates a clear pattern in the microbes that are affected, it seems obvious that these strains compete with one another for resources and a dual approach of reducing the bad guys while increasing the good guys is the best course of treatment.
Good or bad, there will always be competition between bacteria that require the same resources. Co-infections of parasitic organism is very common because some share common food sources, and treating one without treating the other can lead to problems as they may have been keeping each other at bay until you removed the competition for resources. Both are simply trying to survive so competition may prevent one from taking a stronger hold in the body. Beneficial strains of bacteria are known to crowd out pathogenic strains via this competition. Candida albicans is typically a benign strain of yeast found throughout the digestive tract and may even exert some beneficial effects. People with HIV tend to have higher levels of C. albicans and lower levels of a different strain called Pichia while the reverse is seen in healthy people. Candida overgrowth is common in those with HIV, and it turns out that Pichia and C. albicans compete for resources in a petri dish. This indicates that Pichia may help keep C. albicans levels in check by limiting the amount of resources it has to grow.
In addition to competing, parasitic organisms can also team up against you. The fungal form of Candida albicans cannot colonize teeth effectively, but is often found in the oral cavity. On it's own C. albicans won't adhere to teeth, but in the presence of Streptococcus mutans it develops the ability to adhere to teeth as the enzyme that S. mutans uses to metabolize sugar also causes C. albicans to produce a glue-like substance that causes it to bind to S. mutans and adhere to teeth to form plaque. In the presence of both organism, the risk for cavities doubles and the severity of cavities increases seven-fold.
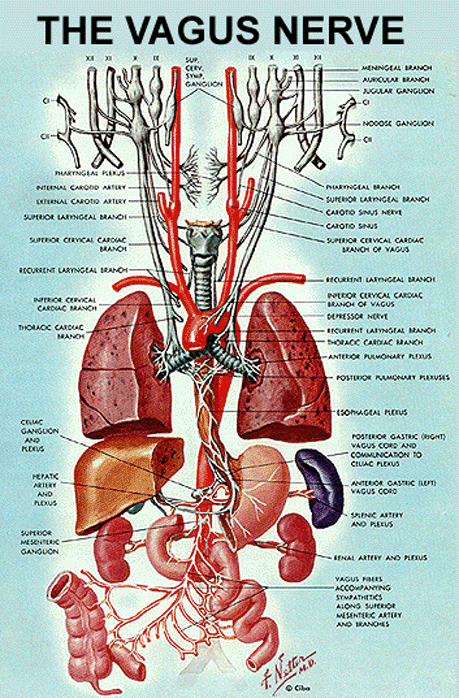
The microbiome is a critical factor in the development of the immune system. Mice raised to be microbiome free have a reduced ability to fight infection as they have a poorly developed immune system with fewer immune cells. This effect is not only seen in the gut where the microbiome exists, but throughout the body as well. Since these are areas where bacteria don't typically exist, it appears that the organisms that make up the microbiome are exerting their effects from the gut on the entire body. The vagus nerve is believed to be the "string" these bacteria are pulling to exert these body-wide effects because when you cut the vagus nerve the beneficial effects of probiotics disappears. When you look at the vagus nerve and where it connects, it makes perfect sense as the vagus nerve connects the brain and gut together. This could also explain why brain and gut health are so closely linked.
It is clear that our understanding of the microbiome is in it's infancy. We are just starting to unravel the effects of specific strains of bacteria on gut and overall health. Lactobacillus rhamnosus is a probiotic strain that has been studied at length and shown to help support those with poorly developed microbiota. It exerts some of it's effects via the production of a protein called p40 that, when attached to receptors of intestinal cells, exerts an anti-inflammatory effect and prevents cell death. While it would be nice to be able to simply take a few probiotic strains to heal many of the conditions that a disturbed microbiota is associated with, it's not that easy.
It's not just which bugs you have, it's how they interact with one another as well. In a study looking at gut bugs and autoantibodies in Type 1 diabetes, researchers found that the microbiome of children who would eventually develop diabetes autoantibodies was similar to that of children who would not, but the bacterial populations within their microbiome interacted differently with one another. This adds yet another layer of complexity in the way the microbiome influences health and shows us that further study is certainly needed.
Overall it looks like the next major step in health and medicine will look at gut health as central to optimal health. The gut is a major interface between the environment and our internal environment, so it makes sense to make sure this barrier does what it's supposed to. One of the keys to optimal gut health is a healthy and diverse microbiome. At this point we have yet to determine exactly what a healthy microbiome is. At this point the best advice is to eat a diverse diet rich in plant material, use antibiotics only when necessary and with probiotics to support your microbiome, and reduce the use of non-steroidal anti-inflammatory drugs. For the health of your children, it is probably best to make every effort to give birth naturally and breast-feed them if at all possible. Eating probiotic foods such as kimchi, sauerkraut, and raw yogurt or kefir if you tolerate dairy may also be beneficial.